Because of the importance of specimen identification, and for establishing protocols for new species boundaries, novel methods and tools for identifying and sharing specimen data for vertebrate organisms, particularly amphibians and reptiles, is an important aim for taxonomists (Dayrat 2005; McDiarmid et al. 2011). In general, the gold standard for specimen collection and identification for reptiles and amphibians is euthanization with appropriate preservation and deposition as vouchered material in natural history holdings (Allmon 1994; Davis 1996; Shaffer et al. 1998; Suarez and Tsutsui 2004; Reynolds and McDiarmid 2011; Simmons 2015). This important approach will rightfully remain the gold standard for collecting and identifying most reptile and amphibian specimens (McDiarmid et al. 2011). However, there is also value in establishing other methods to gain specimen identification as a complement to this method. Several methods already exist (Simmons 2015), including photographs, audio recordings, and scientific illustrations, among others (e.g., https://soundcloud.
com/frogvoicesofborneo). Photographs have proven to be a useful resource for specimen identification and are widely used in online resources such as AmphibiaWeb (amphibiaweb. org). The collection of audio recordings is especially valuable for recording of vocalizations, such as from frogs (e.g., Köhler et al. 2017). Scientific illustrations can be a valuable tool for effective recreation of specimens, especially for emphasizing key elements of scalation and color that might be challenging to document in a photograph. Here, we describe novel tools and techniques for the creation of 3D models of live reptiles and amphibians, both in wild settings in the field and in the laboratory.
Over the last few years, there has been increasing interest in establishing 3D techniques to describe various kinds of specimens, with a focus on museum specimens such as bones, skulls, or other physical features (Aldridge et al. 2005; Chiari et al. 2008; Falkingham 2012; Boyer et al. 2015; Evin et al. 2016; Gignac et al. 2016; Bot and Irschick 2019). The value of 3D scanning methods is their ability to represent high-quality, accurate, shareable, and (typically) complete 3D visualizations of the specimen. Methods for 3D reconstruction include 3D photogrammetry, laser and white-light scanning, and CT- scanning, among others (Weinberg et al. 2004; Gunga et al. 2007; Falkingham 2012; Laforsch et al. 2012). Although widely used for preserved museum specimens, explanation of how these methods can be used for live specimens in the field or the laboratory has been largely unexplored, with some exceptions (Bot and Irschick 2019; Irschick et al. 2020b). However, these methods hold great potential for identifying and cataloguing live reptile and amphibian specimens. This is especially timely given the increasing restrictions placed on scientists striving to access and export reptile or amphibian specimens (Renner et al., 2012). In addition, the software, hardware, and methods for creating and visualizing 3D data have been rapidly evolving over the past decade, and thus represent new opportunities as a powerful visualization tool for specimens.
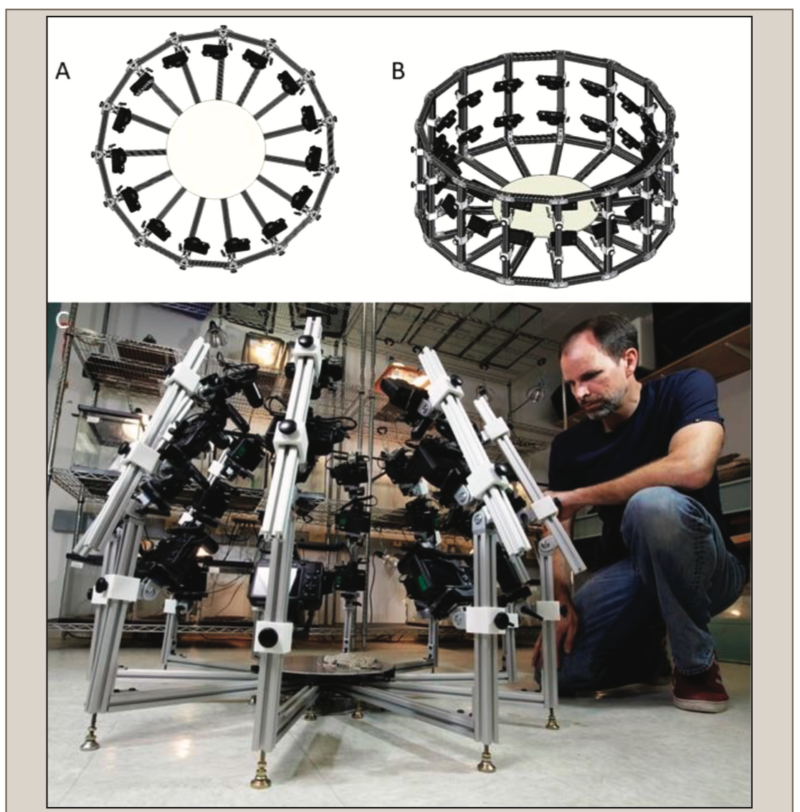
Here, we present novel inexpensive, portable, multi-camera 3D photogrammetry devices that can relatively quickly (1–2 min) create accurate 3D models of live reptile and amphibian species, either in field or laboratory settings. Further processing (several hours) of computer processing time can then result in high-quality RAW 3D models. Finally, an additional input of time (multiple hours) by CG artists can result in full-body 3D meshes for these specimens. 3D photogrammetry is a proven method for accurate recreation of both the shapes and colors of objects using photographs, but while this method is widely used in forensics, art history, paleontology and archaeology, and other scientific applications (Weinberg et al. 2004; Falkingham 2012; Evin et al. 2016), its usage for live animals, including reptiles and amphibians, has hardly been documented (but see Bot and Irschick 2019). We describe the construction of these devices, their usage, and describe the basic workflow of 3D photogrammetry for small reptiles and amphibians both in the field and in the lab. We demonstrate this process by providing examples with 10 species total (four frog species, four lizard species, and two snake species), with five specimens (four frogs, one snake) photocaptured in the field, and five in the laboratory. From these 10 species, we created a total of 11 3D models of which six were RAW (unprocessed) scans, and five were complete (full-body) 3D meshes re-created by CG artists (one species was presented both as a RAW and full-body 3D mesh). By RAW, we mean scans which have not been significantly edited by CG artists and represent initial 3D output. The full-body 3D meshes only show body shape, and not the colors and textures of the species. We demonstrate the relative accuracy of our approach by comparing measurements taken on live individuals with that taken on the digital specimens.
Irschick, Duncan & Corriveau, Zachary & Mayhan, Trevor & Siler, Cameron & Mandica, Mark & Gamble, Tony & Martin, Johnson & Bot, Jeremy & Zotos, Savvas. (2020). Devices and Methods for Rapid 3D Photo-Capture and Photogrammetry of Small Reptiles and Amphibians in the Laboratory and the Field. Herpetological Review. 51. 716-725.